
In the intricate realm of biopharmaceuticals, ensuring the safety and purity of products is paramount. Cobetter, a trailblazer in filtration solutions, continues to push boundaries with its ViruClear Virus Removal lineup, now expanding to offer unparalleled advancements in virus safety strategies.
Empowering Biopharmaceutical Innovation
Virus removal filtration stands as a cornerstone in biopharmaceutical production, safeguarding products like antibodies, recombinant proteins, and blood derivatives against viral contamination. Cobetter’s relentless pursuit of perfection since the launch of its virus removal products in 2021 has led to continuous technological evolution, catering to increasingly complex materials and enhancing filtration capacities.
Diverse Product Offerings
Cobetter’s virus removal filter series encompasses three generations of Polyethersulfone (PES) materials and introduces two new Regenerated Cellulose (RC) products, with Polyvinylidene fluoride (PVDF) options on the horizon. These filters deliver robust retention capabilities, ensuring the efficient removal of endogenous and exogenous viruses, and virus-like particles from diverse proteinaceous materials.
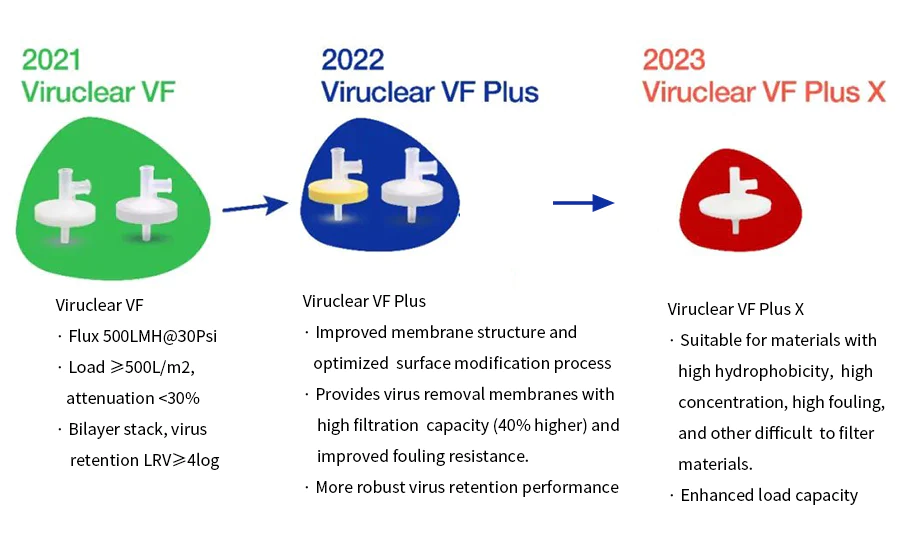
Innovative Solutions for Every Stage
From laboratory-scale process development to large-scale production, Cobetter offers cutting-edge solutions tailored to every stage of biopharmaceutical manufacturing. The upgraded Viruclear® VF WD syringe filters, crafted for process development testing, epitomize Cobetter’s commitment to excellence, providing robust structural stability and enhanced virus removal capabilities.

Optimized Performance with RC Virus Filters
Cobetter’s Regenerated Cellulose (RC) virus removal filters, characterized by superior hydrophilicity and anti-clogging properties, excel in filtration applications for high-protein concentration biologics. The Viruclear® RC (H) series ensures reliable virus removal, even in ultra-high protein environments, underscoring Cobetter’s dedication to precision and efficacy.
Comprehensive Pre-Filtration Solutions
Augmenting its virus removal lineup, Cobetter offers pre-filtration solutions like PNY and PDT filters, designed to enhance efficiency and protect downstream processes. These pre-filters employ advanced pore retention and adsorption mechanisms, ensuring optimal performance across diverse sample compositions.
Integrated Systems for Streamlined Operations
Cobetter’s integrated virus removal filtration systems offer customizable configurations, seamlessly integrating hardware holders and software systems. Equipped with installation points for viral pre-filters, these systems empower users to tailor setups to their specific requirements, optimizing workflow efficiency.

Demonstrated Excellence Through Case Studies
Through experimental case studies, Cobetter showcases the superior performance of its virus removal filters in real-world scenarios. From high initial flux to robust anti-clogging capabilities, Cobetter’s filters demonstrate reliability and consistency, ensuring uninterrupted biopharmaceutical production.
Endorsement of Quality and Compliance
With Drug Master Files (DMFs) formally submitted to the FDA, Cobetter’s viral filtration product series exemplifies adherence to rigorous quality and compliance standards. Cobetter’s extensive support in viral filtration process development and validation further solidifies its reputation as a trusted partner in biopharmaceutical safety.
In conclusion, Cobetter’s expanded ViruClear Virus Removal lineup epitomizes a commitment to excellence, innovation, and safety in biopharmaceutical manufacturing. By harnessing advanced filtration solutions, Cobetter empowers biopharmaceutical companies to navigate complex challenges with confidence, ensuring the integrity and safety of their products.