The biopharmaceutical industry has seen significant growth in recent years, accompanied by stricter validation standards and an enhanced understanding of Quality by Design (QbD) and Process Analytical Technology (PAT). As a result, the need for aseptic sampling has increased, leading to the evolution of closed sampling approaches that now replace conventional techniques like glass vials and Steam In-Place (SIP) sterilization of stainless steel valves.
Regulatory agencies mandate that drug sampling methods not only comply with relevant regulations but also ensure contamination control and drug safety. Here’s an in-depth look at aseptic sampling methods, their regulatory requirements, and the common practices in the industry.
Regulatory Requirements
Compared to the potential cost of pharmaceutical contamination, the expense of sterile sampling is minimal. Regulatory bodies such as the FDA, WHO, and EU GMP, along with industry associations like PDA and PIC/S, provide guidance and recommendations to aid biopharmaceutical companies in transitioning from traditional sampling to aseptic sampling solutions.
211.84 Testing and Approval or Rejection of Components, Drug Product Containers, and Closures
According to this regulation, samples must be collected as follows:
- Containers must be cleaned appropriately before sampling.
- Containers should be opened, sampled, and resealed to prevent contamination.
- Sterile equipment and aseptic techniques are mandatory when necessary.
- Samples from different parts of a container (top, middle, bottom) should not be composited.
- Sample containers must be clearly identified with the material name, lot number, sampling date, and the sampler’s name.
- Containers from which samples have been taken should be marked accordingly.
Common Aseptic Sampling Methods
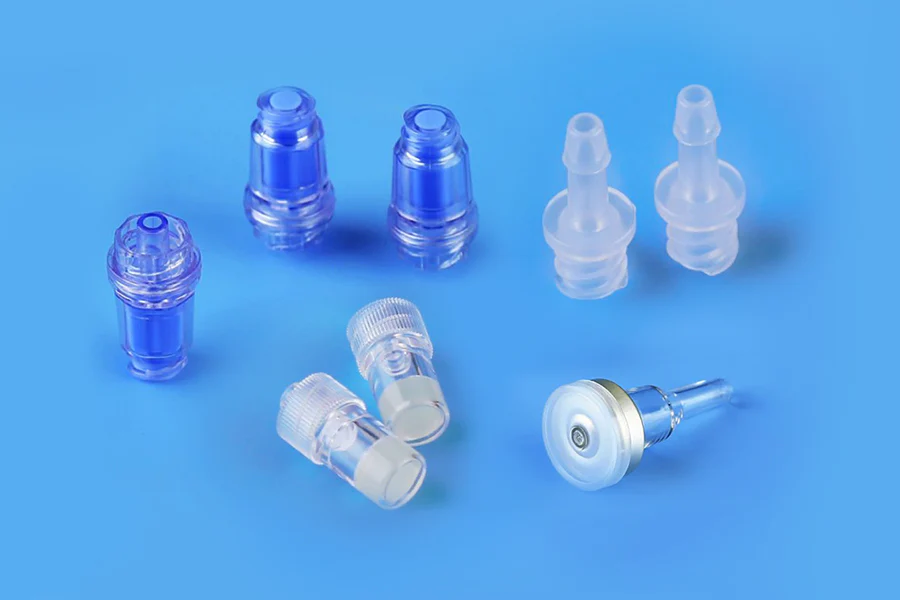
Needlefree Swabable Valve
Features:
- Connects to a matching locking male luer lock fitting to prevent fluid drips or leaks.
- Low pre-charge capacity and high flow.
- Straight-through design for smooth flow.
- Transparent material for easy fluid path viewing.
- Allows repeatable sampling.
Applications:
- Process sampling of bioreactor seed tanks, aseptic dosing, and aseptic post-filtration solutions.
Sampling Plug
Features:
- Uses a sampling needle for puncturing and automatic liquid sealing.
- Sampling by needle syringe.
- Repeatable sampling.
Applications:
- Blood products, medical supplies, cell therapy sampling.
Sampling Plug, ф20mm
Features:
- Provides access and injection port primarily for infusion.
- Enables simultaneous multi-channel sampling using equipment.
- Repeatable sampling.
Applications:
- Bacteria collectors, medical supplies, etc.
Luer Lock
Features:
- Allows sampling by connecting male and female luer connectors.
- Suitable for all types of small-diameter tubing with gas-liquid medium compatibility.
- Can be used with luer syringes.
- Screw design for convenience and safety.
- Repeatable sampling.
Applications:
- Purification, laboratory equipment, biochemical testing, medical supplies, and other rapid, non-sterile sampling operations.
Sampling Needle
Features:
- Matches sampling base, SIP.
- Stainless steel system for in-line sterile sampling.
- Multiple preset sampling paths with the sampling bag.
- Maintains tank or tubing integrity before and after sampling.
- No dead spots or residues at sampling points.
Applications:
- Bioreactor, seed tank, fermenters, formulation tanks, etc.
Cobetter provides safe and versatile sampling solutions that surpass traditional sampling methods. Our joints, supports, and sampling containers are available in various configurations to meet different production processes. We offer comprehensive material screening, functional testing services, and robust verification capabilities to ensure compliance and reduce risks.
Our aseptic sampling solutions ensure representative sample collection in a closed environment, maintaining safety for the environment, product, and operator. Cobetter’s solutions are compatible with stainless steel tanks, single-use tubing, and assemblies, offering convenience and feasibility throughout the entire biopharmaceutical manufacturing process, from culture media preparation to sterile filtration and filling.